Services

Comprehensive Water Treatment Solutions for the Pharmaceutical Industry
Complies with USP, FDA, GMP, and WHO Guidelines.
Overview
We provide complete water treatment solutions for pharmaceutical companies, ensuring high-purity water that fully complies with USP, FDA, GMP, and WHO guidelines. From design to validation, we manage the entire lifecycle of your water systems.


Comprehensive Water Treatment Solutions for the Pharmaceutical Industry
Complies with USP, FDA, GMP, and WHO Guidelines.
Overview
We provide complete water treatment solutions for pharmaceutical companies, ensuring high-purity water that fully complies with USP, FDA, GMP, and WHO guidelines. From design to validation, we manage the entire lifecycle of your water systems.
Our Scope of Services
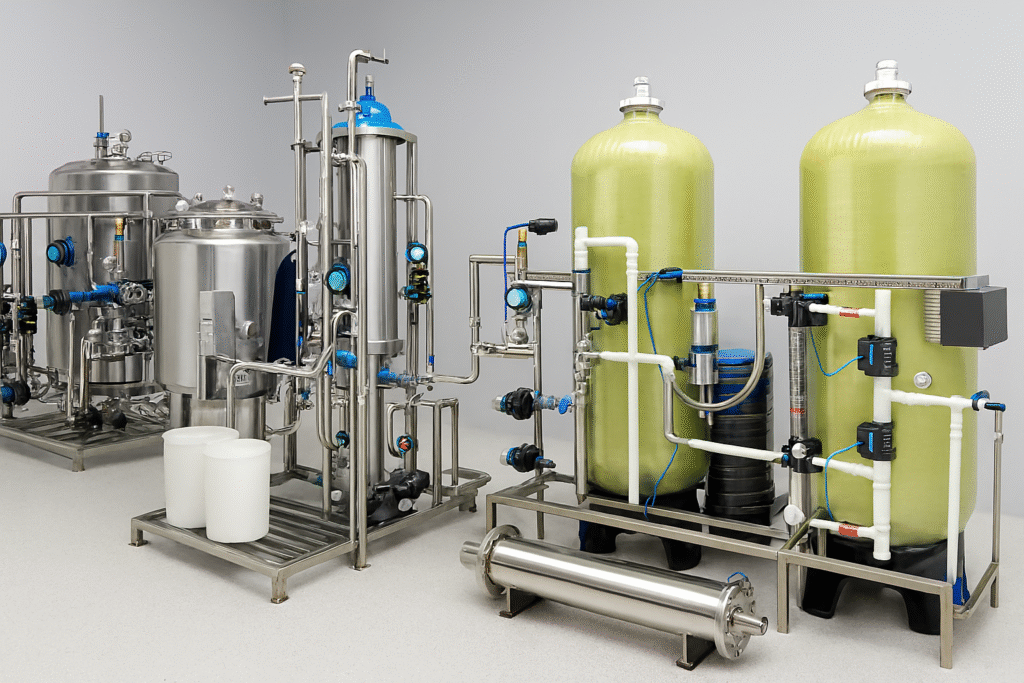
Complete Water Treatment Systems
From aeration to purified water generation, we deliver fully compliant water systems tailored to meet global pharmacopeia standards. Our comprehensive solutions include pre-treatment, reverse osmosis (RO), electrodeionization (EDI), UV disinfection, ozonation, storage, and hygienic distribution systems—ensuring high purity, reliability, and regulatory compliance at every stage.
Supply, Erection & Commissioning
We specialize in the complete supply and commissioning of high-purity water systems, ensuring FDA and GMP-compliant installations with minimal downtime. From initial design to final validation, our experienced team delivers precise execution, full regulatory adherence, and seamless integration into your facility—guaranteeing reliable and compliant water system performance from day one.


Supply, Erection & Commissioning
We specialize in the complete supply and commissioning of high-purity water systems, ensuring FDA and GMP-compliant installations with minimal downtime. From initial design to final validation, our experienced team delivers precise execution, full regulatory adherence, and seamless integration into your facility—guaranteeing reliable and compliant water system performance from day one.

Purified Water Loop Line Extensions
We offer expert design and integration of purified water loop extensions using high-quality SS316L piping, ensuring seamless expansion to new or additional user points. Our systems are built to deliver validated, USP-grade water with full compliance to pharmaceutical standards—supporting both new installations and facility upgrades with precision and reliability.
Documentation & Compliance
We provide comprehensive documentation services to ensure full compliance with USP, FDA, GMP & WHO standards. Our structured and audit-ready documentation supports smooth regulatory inspections and promotes long-term operational reliability—giving you confidence in both system performance and compliance.


Documentation & Compliance
We provide comprehensive documentation services to ensure full compliance with USP, FDA, GMP, and WHO standards. Our structured and audit-ready documentation supports smooth regulatory inspections and promotes long-term operational reliability—giving you confidence in both system performance and compliance.
Our Scope Includes

Design Qualification (DQ)
Comprehensive verification that the design of your water treatment system meets all user requirements and regulatory expectations. Preparation of design review reports, P&IDs, material specifications, and compliance checklists.

Installation Qualification (IQ)
Systematic verification that all equipment has been installed correctly and according to approved design specifications, including verification of materials, utilities, instrumentation, and safety features.

Operational Qualification (OQ)
Validation that the installed system operates as intended under all defined operating conditions, testing control systems, alarms, flow rates, and water quality parameters.

Performance Qualification (PQ) Support
Assistance with PQ protocol preparation, execution, and reporting, ensuring the system consistently produces water of the required purity under actual usage.
Operations & Maintenance
We provide end-to-end Operations & Maintenance (O&M) services, including:
- Preventive and corrective maintenance
- Annual maintenance contracts (AMC)
- Spare parts management
- Operator training for GMP-compliant operation


Operations & Maintenance
We provide end-to-end Operations & Maintenance (O&M) services, including:
- Preventive and corrective maintenance
- Annual maintenance contracts (AMC)
- Spare parts management
- Operator training for GMP-compliant operation
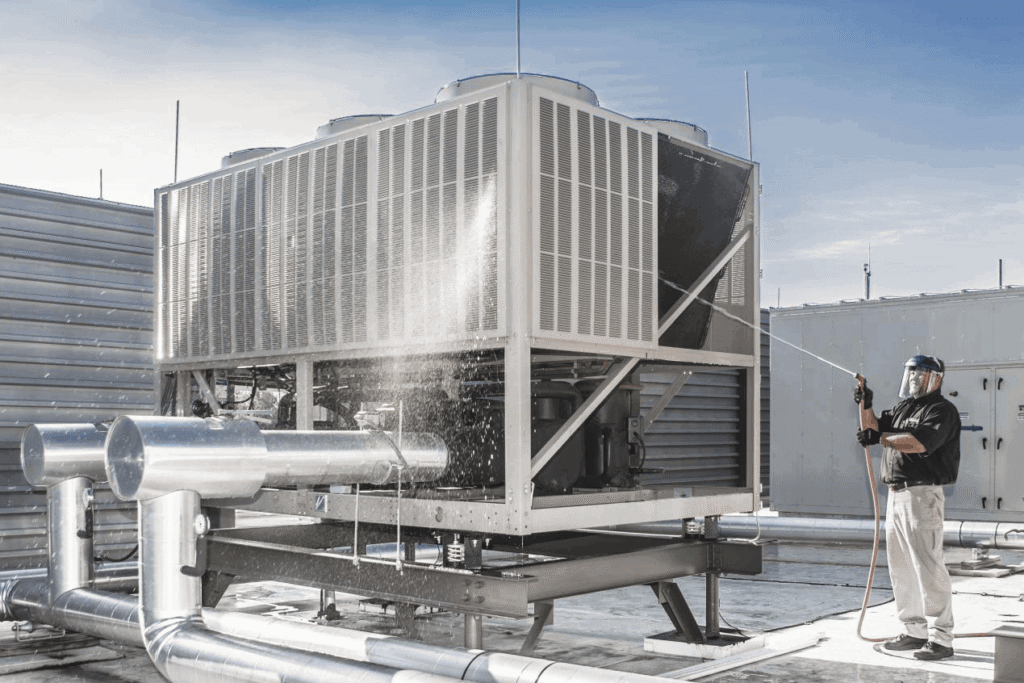
Boiler Cooling Water & Chiller Cooling Water Maintenance
We also specialize in comprehensive maintenance of Boiler Cooling Water and Chiller Cooling Water systems, ensuring optimal performance and energy efficiency.
- Regular chemical treatment and water quality monitoring to prevent scale, corrosion, and biofouling
- Preventive maintenance schedules to minimize downtime
- Troubleshooting and repair of cooling towers, condensers, and chiller pipelines
- Optimization of operating parameters for energy savings and long-term reliability
Troubleshooting & Optimization
We offer rapid diagnosis and effective resolution of issues such as microbial contamination, water quality deviations, and process inefficiencies. Our expert team ensures swift corrective actions to maintain USP and FDA compliance, minimize downtime, and optimize overall system performance.
- Rapid identification and elimination of microbial hotspots through sampling, root cause analysis, and sanitization protocols.
- Immediate corrective actions for deviations in critical parameters like TOC, conductivity, pH, endotoxins, and microbial limits.
- Fine-tuning of RO, EDI, UV, and distribution systems for improved recovery rates, energy efficiency, and reduced chemical usage.


Troubleshooting & Optimization
We offer rapid diagnosis and effective resolution of issues such as microbial contamination, water quality deviations, and process inefficiencies. Our expert team ensures swift corrective actions to maintain USP and FDA compliance, minimize downtime, and optimize overall system performance.
- Rapid identification and elimination of microbial hotspots through sampling, root cause analysis, and sanitization protocols.
- Immediate corrective actions for deviations in critical parameters like TOC, conductivity, pH, endotoxins, and microbial limits.
- Fine-tuning of RO, EDI, UV, and distribution systems for improved recovery rates, energy efficiency, and reduced chemical usage.

Validation Services
Initial and periodic validation services in line with FDA, GMP, and WHO requirements, ensuring regulatory audit compliance and reliable performance.
- Design Qualification (DQ)
- Factory Acceptance Test (FAT)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Why Choose Us -
Pharma-Grade Water Solutions Backed by Expertise
Multi-Industry Expertise
Multi-Industry Expertise Trusted across 10+ sectors
End-to-End Engineering
End-to-End Engineering From concept to compliance
Regulatory Alignment
Regulatory Alignment USP, FDA, GMP, WHO standards
Mission-Critical Support
24/7 technical assistance and rapid supply
-Testimonial-
What Our Clients Says

Mr. Rakesh
Engineering Department, M/s. Umed Pharma Lab Pvt. Ltd, Hyderabad

Mr. Srinivas Reddy
Engineering Department, M/s. Benova Labs Private Limited, Hyderabad

Mr. Radha Krishna
Engineering Department, M/s. Granules India Limited, Hyderabad

Mr. Mallikarjuna
Engineering Department, M/s. Neuland Laboratories Ltd, Hyderabad

Mr. Santosh
Engineering Department, M/s. AET LABORATORIES PRIVATE LIMITED, Hyderabad

Mr. Vishweshwer Muthyam
Engineering Department, M/s. Anvitha Life Care Pvt. Ltd, Hyderabad

Mr. Rakesh
Engineering Department, M/s. Adaarsh Pharmacon Industries Pvt Ltd, Hyderabad

Mr. Sheik Intiaz
Engineering Department, M/s. Lee pharma Limited, Hyderabad
"The Purified Water Storage & Distribution system delivered by your team is truly excellent. The entire setup ...
